Earlier this month, the Massachusetts Department of Health sent a cease-and-desist letter to Good Chemistry, a Colorado-based brand operating in Massachusetts with a dispensary in Worcester and a cultivation facility in Bellingham. The letter claimed Good Chemistry used unapproved pesticides and must close their operations in the state.

According to a Boston Globe article, the company used three pesticides (approved for use on organic food products by the federal government) that cannabis regulators in Nevada, Oregon, Washington and Colorado have all approved for use in cannabis cultivation. Previously, Massachusetts has allowed a number of pesticides to be used on cannabis, but since last year when the state’s Department of Agricultural Resources took over regulating pesticide use on cannabis, they decided to ban all pesticides.
Representatives from Good Chemistry insist the compounds used were safe and that the state is singling them out when the practice is widespread in the industry. “These organic compounds are safe all over the country, and they’re safe in Massachusetts,” Jim Smith, a lawyer for Good Chemistry, tells the Boston Globe. “For the state to single out Good Chemistry for using an industry-standard practice is absolutely wrong. It’s not acceptable — and we’re not going to destroy the crop, because it poses no risk to public safety whatsoever.”

Good Chemistry even disclosed to the state that they would use those pesticides when they applied for a cannabis business license. According to Telegram.com, a local Worcester publication, Matthew Huron, chief executive officer of Good Chemistry, is asking the state to reverse their decision. “The Department of Public Health has the discretion to amend or rescind their order to allow us to make the cannabis we’ve cultivated available to patients in the Worcester community,” says Huron. “Patients have let us know that they really benefited from Good Chemistry’s wide selection of high quality cannabis strains, and they would like access to it again as soon as possible. We’ve asked the state to incorporate the research, analysis and experience that led other states like Colorado, Nevada, Washington and Oregon to determine that the use of these cultivation methods are best practices and helps create healthier, contaminant-free cannabis for patients and the industry as a whole.”
On September 5, the Department of Public Health allowed Good Chemistry to amend the cease-and-desist so they could sell products from other producers in the state. “Many of our patients rely on our medicine we grow specifically and we now are only allowed to sell third party product,” Huron told Telegram.com.





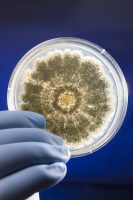



















 According to Susan Bunce, president of DB Labs, ISO accreditation is one way the cannabis lab space is being standardized. “As the first cannabis-testing laboratory in Nevada, DB Labs has always taken patient safety very seriously and has always tried to raise the bar,” says Bunce. “The world of cannabis testing is often compared to the Wild West: each lab uses state regulations to set their standards, but it leaves a lot of room for subjective interpretations. The ISO accreditation removes the ambiguity and guarantees a consistent level of testing to users. We are proud to be a part of that.”
According to Susan Bunce, president of DB Labs, ISO accreditation is one way the cannabis lab space is being standardized. “As the first cannabis-testing laboratory in Nevada, DB Labs has always taken patient safety very seriously and has always tried to raise the bar,” says Bunce. “The world of cannabis testing is often compared to the Wild West: each lab uses state regulations to set their standards, but it leaves a lot of room for subjective interpretations. The ISO accreditation removes the ambiguity and guarantees a consistent level of testing to users. We are proud to be a part of that.”








