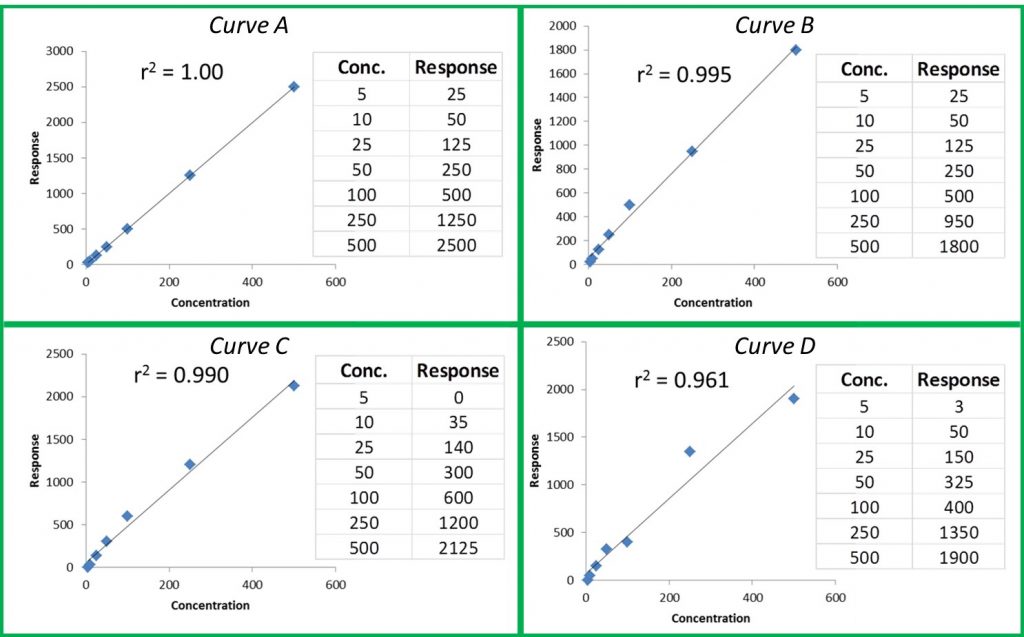
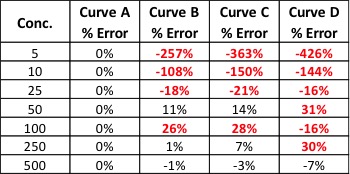
In the last article I referred to the analogy of the analytical reference material being a keystone of the laboratory foundation, the stone upon which all data relies. I then described the types of reference materials and their use in analytical testing in general terms. This article will describe the steps required to properly manufacture and deliver a certified reference material (CRM) along with the necessary documentation.
A CRM is an exclusive reference material that meets strict criteria defined by ISO Guide 34 and ISO/IEC 17025. ISO is the International Organization for Standardization and IEC is the International Electrotechnical Commission. These organizations work together to set globally recognized standards. In order for a reference material to be labeled as a CRM it must 1) be made with raw or starting materials which are characterized using qualified methods and instruments, 2) be produced in an ISO-accredited lab under documented procedures, and 3) fall under the manufacturer’s scopes of accreditation. Verifying a CRM supplier has these credentials is easily done by viewing their certificates which should include their scopes of accreditation. 
There are many steps required to produce a CRM that meets the above three criteria. The first step requires a review of the customer’s, or end-user’s requirements to carefully define what is to be tested, at what levels and which analytical workflow will be used. Such information enables the producer to identify the proper compounds and solvents required to properly formulate the requested CRM.
The next step requires sourcing and acquiring the raw, or starting materials, then verifying their compatibility and stability using stability and shipping studies in accordance with ISO requirements. Next the chemical identify and purity of the raw materials must be characterized using one or more analytical techniques such as: GC-FID, HPLC, GC-ECD, GC-MS, LC-MS, refractive index and melting point. In some cases, the percent purity is changed by the producer when their testing verifies it’s different from the supplier label. All steps are of course documented.
 The producer’s analytical balances must be verified using NIST traceable weights and calibrated annually by an accredited third party provider to guarantee accurate measurement. CRMs must be prepared using Class A volumetric glassware, and all ampules and vials used in preparation and final packaging must be chemically treated to prevent compound degradation during storage. Next, CRMs are packaged in an appropriate container, labeled then properly stored to maintain the quality and stability until it’s ready to be shipped. All labels must include critical storage, safety and shelf life information to meet federal requirements. The label information must be properly linked to documentation commonly referred to as a certificate of analysis (COA) which describes all of the above steps and verifies the traceability and uncertainty of all measurements for each compound contained in the CRM.
The producer’s analytical balances must be verified using NIST traceable weights and calibrated annually by an accredited third party provider to guarantee accurate measurement. CRMs must be prepared using Class A volumetric glassware, and all ampules and vials used in preparation and final packaging must be chemically treated to prevent compound degradation during storage. Next, CRMs are packaged in an appropriate container, labeled then properly stored to maintain the quality and stability until it’s ready to be shipped. All labels must include critical storage, safety and shelf life information to meet federal requirements. The label information must be properly linked to documentation commonly referred to as a certificate of analysis (COA) which describes all of the above steps and verifies the traceability and uncertainty of all measurements for each compound contained in the CRM. 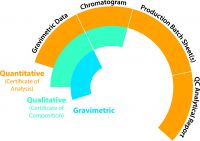
My company, RESTEK, offers a variety of documentation choices to accompany each CRM. Depending on the intended use and data quality objectives specified by the end-user, which were defined way back at the first step, three options are typically offered: They include gravimetric only, qualitative which includes gravimetric, and fully quantitative which includes all three levels of documentation. The graphic to the right summarizes the three options and what they include.
It’s important to understand which level you’re purchasing especially when ordering a custom CRM from a supplier. Most stock CRMs include all three levels of documentation, but it’s important to be sure.
Understanding what must be done to produce and deliver a CRM sets it apart from other reference material types, however it’s important to understand there are some instances where CRMs are either not available, nor required and in those situations other types of reference materials are perfectly acceptable.
If you have any questions or would like more details about reference materials please contact me, Joe Konschnik at (800) 356-1688 ext. 2002 by phone, or email me at joe.konschnik@restek.com.