Parts One and Two in this series have defined Good Manufacturing Practices, introduced Hazard Analysis and Critical Control Points (HACCP) and explained the first HACCP step of hazard analysis. A food safety team will typically work from a flow diagram to identify biological, chemical or physical hazards at each step of processing and packaging. Once the hazard is identified, the severity and probability are debated. Hazards with severe consequences or high probability are carried through the HACCP plan as Critical Control Points (CCPs).
Critical Control Points definedHACCP is a do-it-yourself project.
Where exactly will the hazard be controlled? CCPs are embedded within certain steps in processing and packaging where the parameters, like temperature, must be met to ensure food safety. Failure at a CCP is called a deviation from the HACCP plan. The food safety team identifies where manufacturing problems could occur that would result in a product that could cause illness or injury. Not every step is a CCP! For example, I worked with a client that had several locations for filters of a liquid stream. The filters removed food particles, suspended particulates and potentially metal. We went through a virtual exercise of removing each filter one-by-one and talking through the result on controlling the potential hazard of metal. We agreed that failure of the final filter was the CCP for catching metal, but not the other filters. It was not necessary to label each filter as a CCP, because every CCP requires monitoring and verification.
Identification of a CCP starts more documentation, documentation, documentation.
Do you wish you had more reports to write, more forms to fill out, more data to review? No. Nobody wants more work. When a CCP is identified, there is more work to do. This just makes sense. If a CCP is controlling a hazard, you want to know that the control is working. Before I launch into monitoring, I digress to validation.
CCP validationThis is where someone says, “We have always done it this way, and we have never had a problem.”
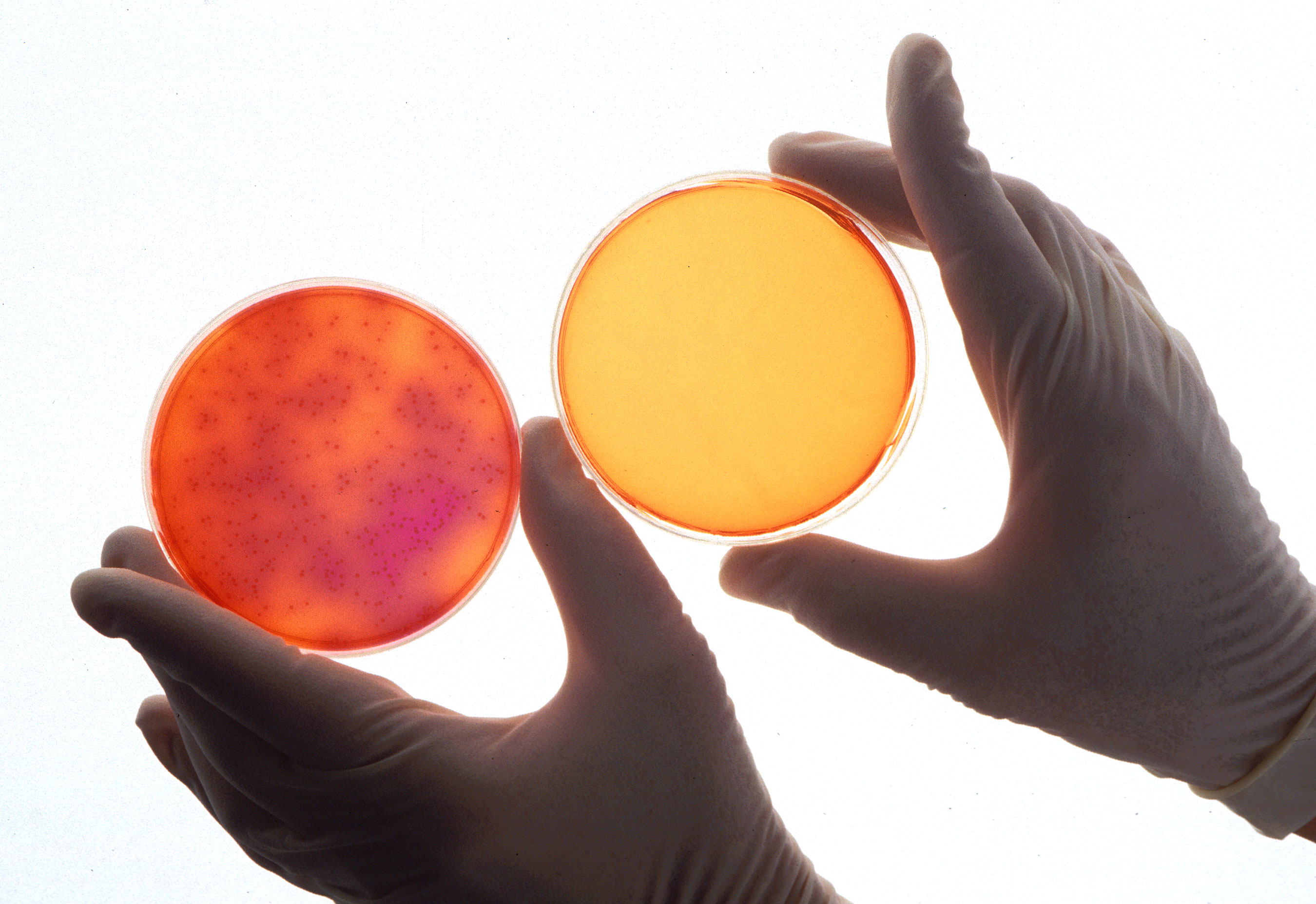
You want to know if a critical step will actually control a hazard. Will the mesh of a filter trap metal? Will the baking temperature kill pathogens? Will the level of acid stop the growth of pathogens? The US had a major peanut butter recall by Peanut Corporation of America. There were 714 Salmonella cases (individuals) across 46 states from consumption of the contaminated peanut butter. Imagine raw peanuts going into a roaster, coming out as roasted peanuts and being ground into butter. Despite the quality parameters of the peanut butter being acceptable for color and flavor, the roasting process was not validated, and Salmonella survived. Baking of pies, pasteurization of juice and canning all rely on validated cook processes for time and temperature. Validation is the scientific, technical information proving the CCP will control the hazard. Without validation, your final product may be hazardous, just like the peanut butter. This is where someone says, “We have always done it this way, and we have never had a problem.” Maybe, but you still must prove safety with validation.
The hazard analysis drives your decisions.
Starting with the identification of a hazard that requires a CCP, a company will focus on the control of the hazard. A CCP may have one or more than one parameter for control. Parameters include time, temperature, belt speed, air flow, bed depth, product flow, concentration and pH. That was not an exhaustive list, and your company may have other critical parameters. HACCP is a do-it-yourself project. Every facility is unique to its employees, equipment, ingredients and final product. The food safety team must digest all the variables related to food safety and write a HACCP plan that will control all the hazards and make a safe product.
Meeting critical limits at CCPs ensures food safety
The HACCP plan details the parameters and values required for food safety at each CCP.The HACCP plan identifies the minimum or maximum value for each parameter required for food safety. A value is just a number. Imagine a dreadful day; there are problems in production. Maybe equipment stalls and product sits. Maybe the electricity flickers and oven temperature drops. Maybe a culture in fermentation isn’t active. Poop happens. What are the values that are absolutely required for the product to be safe? They are often called critical limits. This is the difference between destroying product and selling product. The HACCP plan details the parameters and values required for food safety at each CCP. In production, the operating limits may be different based on quality characteristics or equipment performance, but the product will be safe when critical limits are met. How do you know critical limits are met?
CCPs must be monitored
Every CCP is monitored. Common tools for monitoring are thermometers, timers, flow rate meters, pH probes, and measuring of concentration. Most quality managers want production line monitoring to be automated and continuous. If samples are taken and measured at some frequency, technicians must be trained on the sampling technique, frequency, procedure for measurement and recording of data. The values from monitoring will be compared to critical limits. If the value does not reach the critical limit, the process is out of control and food safety may be compromised. The line operator or technician should be trained to know if the line can be stopped and how to segregate product under question. Depending on the hazard, the product will be evaluated for safety, rerun, released or disposed. When the process is out of control, it is called a deviation from the HACCP plan.
A deviation initiates corrective action and documentation associated with the deviation. You can google examples of corrective action forms; there is no one form required. Basically, the line operator, technician or supervisor starts the paperwork by recording everything about the deviation, evaluation of the product, fate of the product, root cause investigation, and what was done to ensure the problem will not happen again. A supervisor or manager reviews and signs off on the corrective action. The corrective action form and associated documentation should be signed off before the product is released. Sign off is an example of verification. Verification will be discussed in more detail in a future article.
My thoughts on GMPs and HACCP were shared in a webinar on May 2nd hosted by CIJ and NEHA. Please comment on this blog post below. I love feedback!